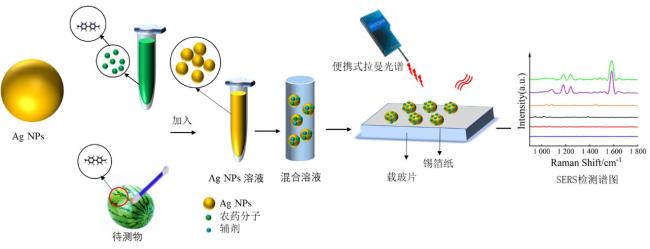
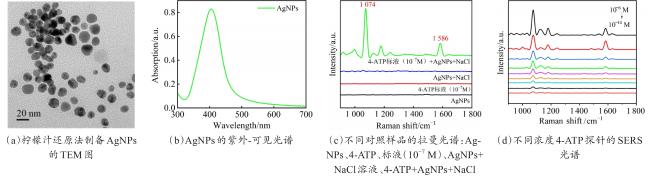
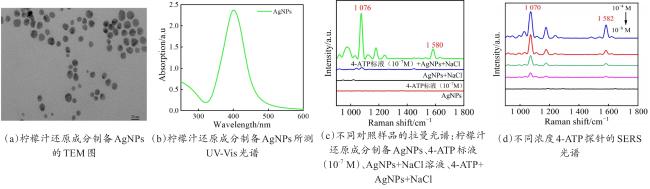
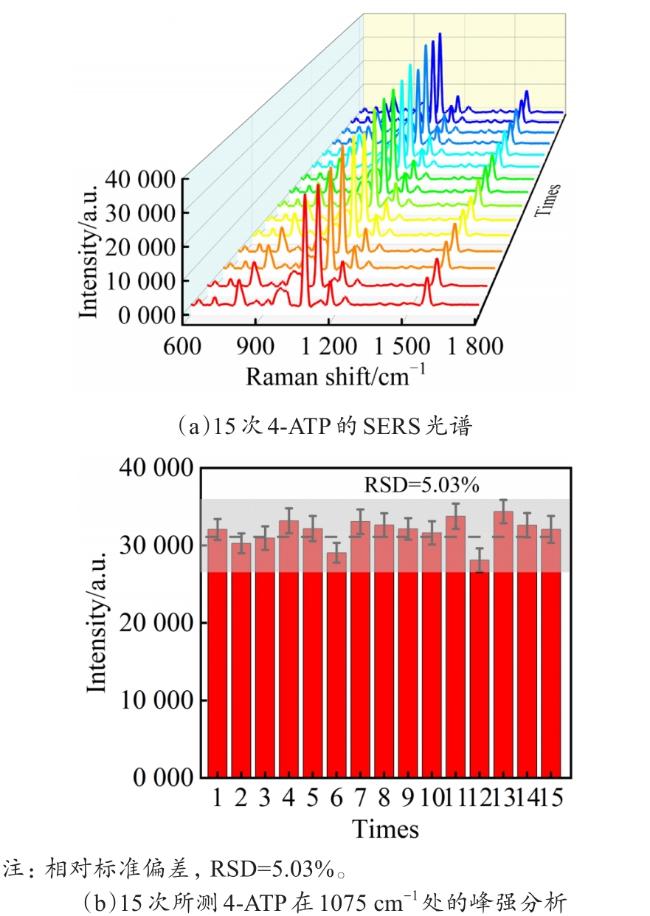
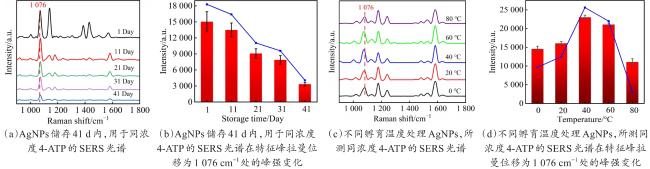
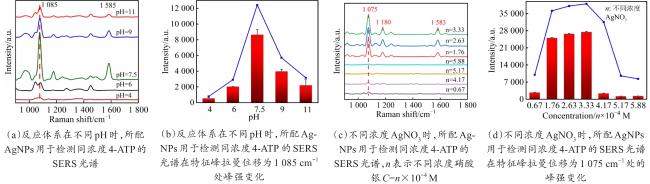
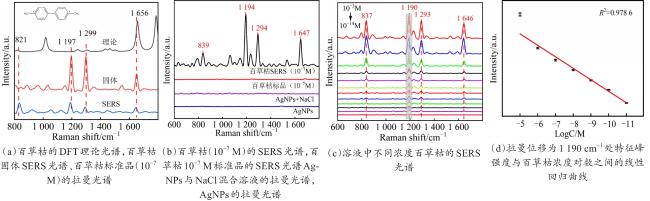
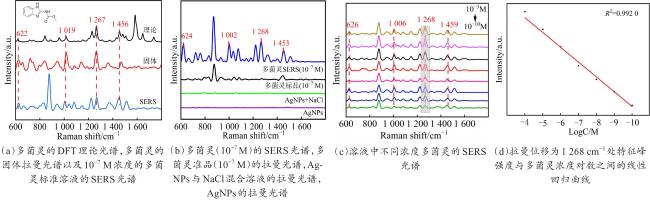
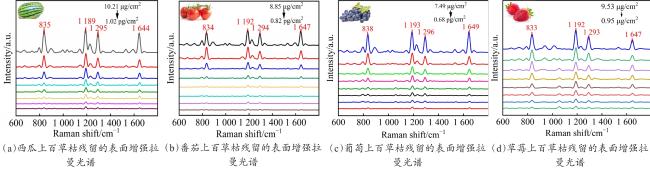
Objective The use of pesticides is one of the root causes of food safety problems. Pesticide exposure and pesticide residues can not only lead to environmental pollution issues but also seriously affect human health. In order to meet the rapid and sensitive detection needs of pesticide residues in agricultural products, a method based on lemon juice reduction to prepare silver nanoparticles (AgNPs) is reported in this research. Methods First, fresh lemon juice was filtered through filter paper and diluted to a 2% lemon juice aqueous solution. Then, a certain concentration of AgNO3 solution, 50 mm NaOH solution were prepared and stored at room temperature. Then, 10 mL ddH2O, 2 mL NaOH, 2 mL 2% lemon juice, and 5 mL AgNO3 solution were mixed. When the solution turned to a clear yellow color, the solution was centrifuged to obtain AgNPs. The morphology and structure of AgNPs were observed by transmission electron microscopy (TEM). In order to verify the successful synthesis of the nanoparticles and the distribution characteristics of the nanoparticles, ultraviolet spectroscopy was used for measurement and analysis, and 4-ATP was used as a SERS probe to preliminarily verify the SERS enhancement performance of AgNPs. Furthermore, the content of the main reducing components in lemon juice, namely ascorbic acid, glucose, and fructose was analyzed. The content of ascorbic acid in lemon juice was determined by high-performance liquid chromatography, and the content of glucose and fructose in lemon juice was determined by UV-visible spectrophotometry. To verify the stability and uniformity of the SERS signal of the nanoparticles, 4-ATP was used as an surface enhancement of raman scattering (SERS) probe for detection analysis. The stability of the SERS performance of the colloidal substrate within 41 days and the SERS performance at temperatures ranging from 0-80 °C were analyzed. Using 4-ATP as the SERS probe, the experimental conditions were optimized for the preparation of AgNPs by the lemon juice method, including pH and AgNO3 concentration. To validate the practical usability of the nanoparticles, the solutions of paraquat and carbendazim and the detection limits of pesticide residues on different fruits and vegetables were detected by SERS. Results and discussions The method for preparing AgNPs has the advantages of simple operation, green and easy synthesis. The particle morphology and size of the prepared AgNPs were basically uniform, with a size of about 20 nm. The ultraviolet-visible spectrum of AgNPs solution showed that the absorption peak was about 400 nm and the peak shape was narrow, indicating that the colloidal solution had good homogeneity. The detection limit of 4-ATP as the SERS probe was 10-14 M, indicating that the nanoparticle had a good SERS. In addition, the content of ascorbic acid, the main reducing ingredient, in lemon juice measured by high-performance liquid chromatography (HPLC) was 395.76 μg/mL. The contents of glucose and fructose, which were the main reducing components in lemon juice, were 5.95 and 5.90 mg/mL, respectively. Furthermore, the characterization and analysis results of the AgNPs prepared by the mixed reducing solution prepared according to the concentration data of each component showed that the AgNPs obtained were also uniform in morphology and size, with a diameter of about 20 nm, but the SERS enhancement performance was not as good as that of the AgNPs reduced by lemon juice. The SERS signal uniformity of the AgNPs reduced by lemon juice analyzed results showed that the peak intensity of the SERS spectral of 4-ATP at different sites at the same concentration was not significantly changed for 15 times, and its standard deviation RSD=5.03%, which was much lower than the intersubstrate RSD value (<16%) of the qualified new SERS active substrate for quantitative analysis. The temporal stability and temperature stability of AgNPs analyzed results showed that the nanoparticles still had SERS enhanced performance after 41 days of storage, and had SERS enhanced performance stability over a wide temperature range (0~80 °C). In addition, the optimization results of experimental conditions showed that the optimal pH for the preparation of AgNPs was around 7.5, and the optimal range of AgNO3 concentration used was 1.76×10-4~3.33×10-4 mol/L. Finally, using AgNPs prepared by lemon juice reduction method for pesticide residue SERS detection on the surface of fruits and vegetables, the detection limits for paraquat and carbendazim in solution were as low as 10-14 and 10-10 M, respectively, and the concentration of pesticides showed a good linear relationship with Raman spectral intensity. The lowest detection limits for paraquat and carbendazim residues on different fruits and vegetables were as low as 3.90 ng/kg and 0.22 µg/kg, respectively. Conclusions This work provides a green and convenient method for preparing SERS materials for rapid detection of pesticide residues on fruits and vegetables. This method has practical value for universal operation. The prepared AgNPs can be used for trace pesticide residue detection, providing a pathway for rapid and sensitive detection of pesticide residues in agricultural products.